Research
Lymphocyte synapse and polarity
When a lymphocyte encounters another cell…
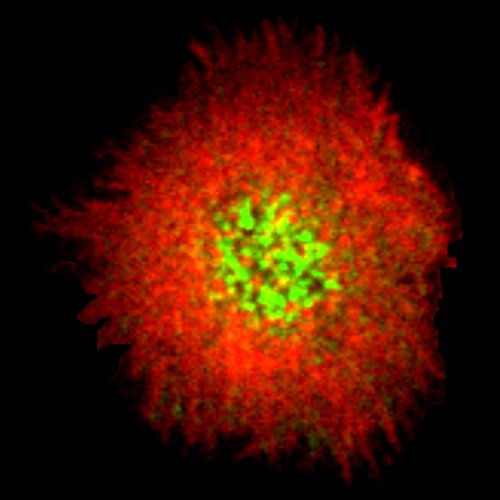
B and T cells recognize the antigen thanks to their specific receptor and form a special signaling platform (the immune synapse) when in contact with a so called “antigen presenting cell”. After synapse formation, the lymphocyte accumulates the antigen at the center of the contact and reorganizes its organelles to polarize, ultimately enabling to perform its immune function (antigen extraction and processing for B cell, killing for cytotoxic T cells, activation for CD4 T cells). Understanding what controls the dynamics of immune synapse formation, the polarization of the cell and the response of the immune cells is therefore a crucial step in improving or modulate antibody production (B cells), T cell activation (CD4 T cells) and target killing (cytotoxic T cells and CAR-T).
The kiss of death…
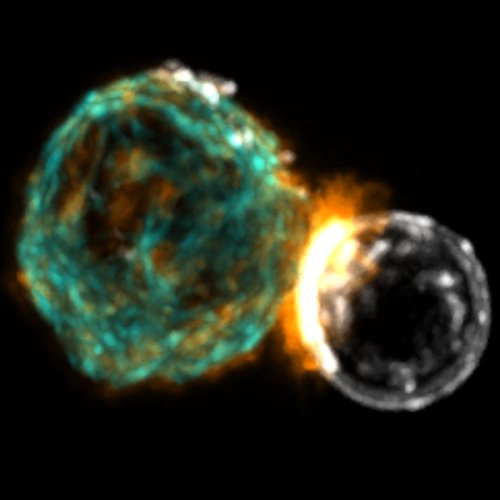
Several cell types communicate with each other through a signaling platform (called synapse) that allows passage of molecules and signals. In particular, B cells form an **immune synapse** with cells exposing antigens at their surface. Traditionally the immune synapse is studied in vitro by plating the cells on functionalised surfaces. This allows us to study what happens inside the cell but the antigen is anchored on the glass coverslip and cannot move. On lipid bilayer, as the surface is fluid, we can follow the formation of the synapse at single molecule level. We also use photoconvertible fluorescent protein to study the movement of molecular motors (Myosin II) at the synapse (SPT-PALM).
Microfluidics
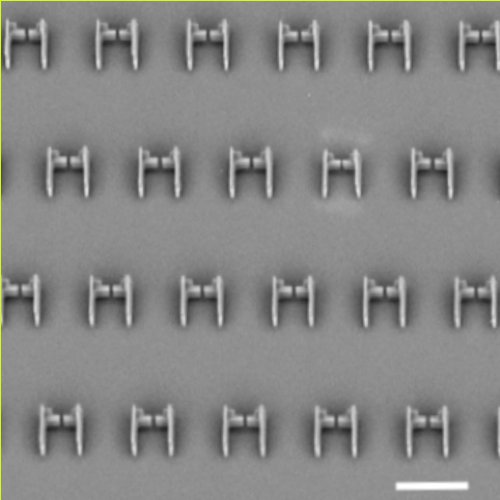
To study what happen to the B or T cell when it interacts with the antigen presenting cells, we introduce a pairing cell **microfluidic chip** that allows multiple simultaneous observations of synapse formation. To modulate the different physico-chemical properties of the presenting cell, we develop and characterize a new material, the lipid droplet, whose features (size, surface tension, fluidity, ligand concentration) can be easily controlled and that can be used as force sensor. By presenting the antigen on functionalised oil droplets and trapping doublets (droplets – B cell) in microfluidic traps we could follow multiple polarization events and investigate the polarization kinetics (what comes first?) and the interplay between actin and microtubules networks (what controls what?) in the formation of a functional synapse. Using quantitative live imaging, we can characterize the movement of most major actors upon antigen recognition from the first contact, and the impact of drugs targeting the cytoskeleton on their polarization dynamics. We will apply this new set of experimental and analytical tools to investigate the polarization and signaling dynamics of differnet cells. This can also open up perspective in understanding the fundamental processes used by cells in recent cancer immunotherapies.
Mechanics
TFM: Use the force young B cell!
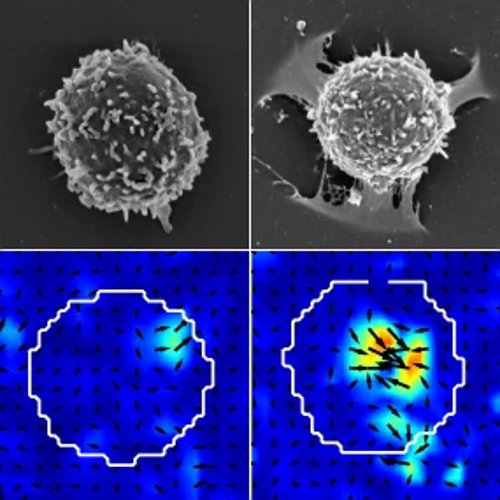
B cells initiate humoral adaptive immune response by recognizing the antigen presented at the surface of another cell. We study by traction force microscopy (TFM) the force exerted by the cell during this process. This techniques consists in observing the deformations of a soft gel induced by a cell plated on it. We observe peculiar spatio-temporal patterns in the forces and show that actin protrusions at the center of the synapse are the main site of antigen extraction and endocytosis.
What does a cell feel inside a living lymph node?
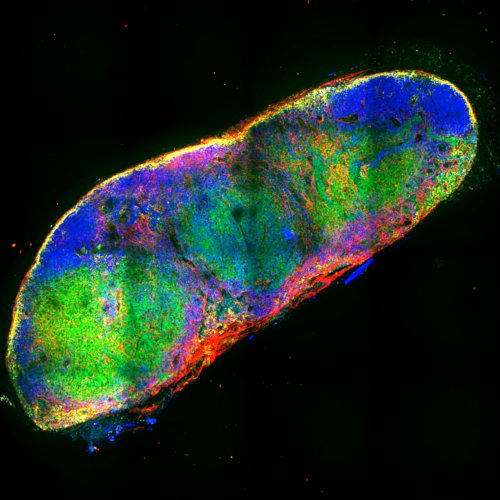
Formation of the synapse is critical for many immune cells in order to mechanically extract the antigen, recongise cognate antigen, recognise targes to kill etc. We hypothesize that the structure of the synapse and the forces generated therein are essential for its **mechanical stability** in the physiological conditions of the lymph node, whereby the synapse is perturbed by external flows and non-specific cell-cell interactions. We are testing this hypothesis by a combination of in situ, in vitro and in silico approaches. The project aims at a larger comprehension of how tissue mechanics influence biological cell functions.
We propose first to measure the mechanical perturbations a cell is subject to in the lymph node. The measure of the mechanic and the mechanical activity of the living tissue are performed through microrheology techniques, that include observing cells and particles in living tissue slices. By using deformable lipid droplets we will estimate the stress the cells undergo and the time scale of their movement and deformations. Tissue slices allow access to the a living systems for hours, and to perturb it mechanically or chemically.
Mechanical perturbation of the immune synapse
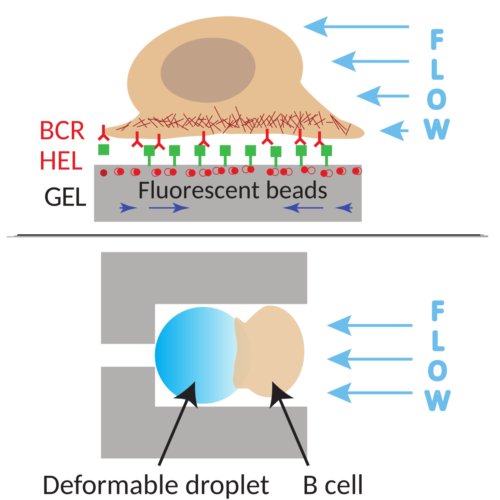
To understand how the mechanics of the lymph node affect the functions of immune cells, in particular B cells, we will characterize the morphology, mechanics, and functionality of the synapse under perturbing hydrodynamics flow in microfluidic chips. We will further estimate the impact at the sub cellular level using in vitro microfluidic approaches to monitor by dynamic imaging the effect of compression and shearing on the cells and simultaneously measure the forces applied by the cells by traction force microscopy.
Criticality in lymphocytes activation
Is the lymphocyte on the verge of criticality?
Stay tuned!!
Cytoskeleton and inflammation
Stay tuned!!